This Article
Citations

Except where otherwise noted, this work is licensed under Creative Commons Attribution-NonCommercial 4.0 International License.
Myocardial Deformation Indices in Patients with Significant Aortic Regurgitation: A Strain Rate Imaging Study
Abstract
Background: Early detection of myocardial contractility disturbances in patients with asymptomatic moderate to severe aortic regurgitation (AR) leads to the early operative intervention early enough to prevent from poor postoperative heart failure (HF) prognosis.
Objective: Our aim was to study the subclinical left ventricular (LV) dysfunction, by novel echocardiographic methods, myocardial deformation indices in patients with asymptomatic, and significant aortic regurgitation (AR).
Patients and Methods: Standard echocardiogram and complementary tissue Doppler imaging (TDI) and Doppler based strain and strain rate (S/SR) imaging were performed in 44 asymptomatic patients with pure and significant AR and ejection fraction (EF) more than 50% (mean age:49.9 ± 17.2 years, 50% male) and 20 healthy participants (mean age: 47.3 ± 13.8 years, 65% male). In addition to TDI velocities to investigate the LV longitudinal deformation, peak systolic S and SR were measured at septal, lateral and posterior walls. The LV modified myocardial performance index (MPI) or Tie index also were calculated via TDI study.
Results: AR group had significantly increased LV end systolic and end diastolic volumes, interventricular septum and posterior wall thickness compared to controls. In AR, Sm and Em of septal wall, Sm of lateral wall; S and SR of both septal and lateral walls were significantly decreased while MPI were significantly increased compared to healthy participants.
Conclusions: Our results demonstrated the ability of PW-TDI and S/SR modalities in detection of the early subclinical abnormalities in asymptomatic patients with significant chronic AR.
Keywords: Aortic Regurgitation; Tissue Doppler Imaging; LV Velocity; Strain; Strain Rate; Subclinical LV
1. Background
According to the previous studies, in aortic regurgitation (AR), changes in regurgitated volume or increases in left ventricular (LV) systolic or diastolic pressure can cover underlying abnormal changes in myocardial force development due to the myocardial damage. It is challenging to determine the subclinical myocardial abnormalities and also the optimum time for operative intervention in asymptomatic patients with chronic significant AR. It is important not to subject patients to the early and unnecessary operative risks and morbidity related to prosthetic valves (1) and postpone the operation until irreversible LV systolic dysfunction occurs (2-6). In the initial process of AR, many patients are asymptomatic since compensatory mechanism such as LV eccentric hypertrophy keep the LV ejection fraction (EF) in the normal ranges (7) with progressive LV dilation, contractility impairment would occur, however , all of the symptoms are not related to developing LV dysfunction (8). Therefore, early detection of subclinical LV systolic dysfunction is crucial and could influence patients' prognosis by aiding the clinician to candidate patients for better management (9-12). As well, conventional echocardiography indices may not show any subclinical systolic dysfunction at the early stage of myocardial irreversible damage (13). There is still no consensus on measurement variables which could absolutely discriminate the subclinical LV systolic dysfunction in patients with chronic AR; however, EF and LV end-systolic diameter (ESD) are the two most widely utilized methods to evaluate LV function (6, 14) and the surgery is recommended to be performed on the asymptomatic severe AR patients before the ejection fraction (EF) falls below 50% and LVESD exceeds 55mm (15). As these parameters are indirect measurements of myocardial function, they could only evaluate late hemodynamic consequence of AR and are not too able to detect subclinical LV systolic dysfunction. Thus there is a need to more sensitive tool which could detect myocardial damage at its initial phase. Recently, pulsed-wave tissue Doppler imaging (PW-TDI) and also 2D or Doppler based strain (S)strain rate (SR) derived parameters have been shown to be useful noninvasive tools for detecting subtle LV contractile changes in the preceding reduced LVEF and markedly dilated LV in patients with AR (6, 8, 10). TDI derived regional velocities are especially useful for detection of initial subendocardial changes in longitudinal myocardial contraction that would deteriorate in primary stage and subtle systolic dysfunction and also calculation of MPI which is load independent index of LV systolic and diastolic functions (8, 10). Furthermore, strain and strain rate imaging are relatively more accurate newer techniques for quantification of global and regional contractile dysfunction in subclinical cases (8, 10, 16-18). In the current study, we hypothesized that there is a need in the current literature to investigate the early changes of these new indices in asymptomatic patients with moderate or severe AR. Therefore we analyzed the LV regional myocardial velocities and deformity indices in asymptomatic patients with moderate and also more than moderate chronic AR and preserved LVEF to compare these parameters with those of healthy population. As there has not yet been enough studies evaluating the reliability of these new echocardiogrphic indices, it is need to conduct more specific studies determining the reliable echocardiographic cut-offs for clinical applications in future.
2. Objective
Our aim was to study subclinical left ventricular (LV) dysfunction, by novel echocardiographic methods, myocardial deformation indices in patients with asymptomatic, significant aortic regurgitation (AR).
3. Patients and Methods
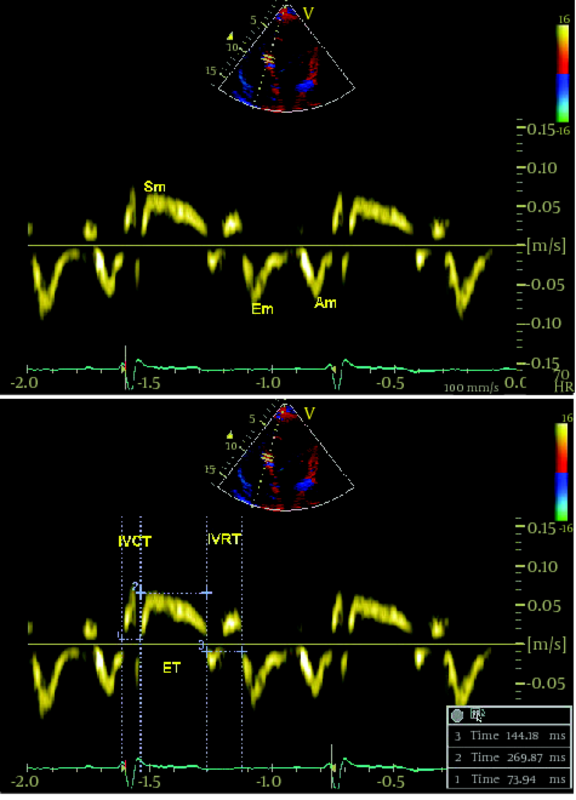
Among the patients who referred to the Echocardiographic Department at Tehran Heart Center between October 2009-March 2010, due to suspicious cardiac sounds or as a part of medical checkup, we included 44 asymptomatic patients (mean age ± SD: 49.9 ± 17.2 years, 50% male) with a diagnosis of isolated chronic AR with any etiology (Rheumatismal, Bicuspid or degenerative) who were asymptomatic and had moderate or more than moderate degree AR, determined by echocardiographic criteria according to published American Society of Echocardiography(ASE) guidelines (19), who did not meet the study exclusion criteria as follows: 1) coexisting other valvular diseases more than mild degree, 2) non-sinus cardiac rhythm, 3) LV ejection fraction < 50% , 4) left ventricular end systolic diameter(LVESD) > 55mm or left ventricular end diastolic diameter (LVEDD) > 75mm, 5) any known or suspected coronary artery disease based on electrocardiogram or angiogram or having major risk factors, 6) previous cardiac surgery, 7) low quality echocardiographic image for TDI or S/SR analyses.During the same period, 20 healthy controls (mean age ± SD: 47.3 ± 13.8 years, 65% male) have been enrolled from the hospital staff via advertisements using announcements in the local media. This control population did not have any symptom or history of heart diseases, systemic hypertension, or diabetes mellitus and had normal findings of electrocardiography and conventional echocardiography at rest. Informed consent was obtained from each patient and the study protocol was approved by local Ethics Committee of our institution. A combination of standard transthoracic, PW-TDI and study of longitudinal SR and S were performed by the use of commercially available ultrasound system (Vivid 7, Vingmed GE, Horten, Norway, 3.5-MHz transducer). All patients were examined at rest in left lateral decubitus position. Measurements were made according to the ASE guidelines. By the use of M-mode study, left ventricular end-diastolic (LVEDD) and left ventricular end-systolic (LVESD) diameters , left atrium (LA) diameter, interventricular septum (IVS) and posterior wall (PW) thickness were measured from parasternal long-axis view. From the apical four chambers view, left ventricular end-diastolic (LVEDV) and end-systolic volumes (LVESV) and LV EF were calculated using modified Simpson’s method. AR severity was assessed by measuring the width of the regurgitated jet at its ratio to the LVOT diameter and the narrowest height of AR jet at the level of aortic valve in color Doppler flow study (VC) from the parasternal long-axis view. PW-TDI study was set for a frame rate between 120 and 180 Hz, and a cine loop of three consecutive heart beats. The 2.5mm TDI sample volume was placed at the junction of the LV wall with the mitral annulus of the septal and lateral myocardial segments from the four chamber view. Efforts were done to obtain the best angle (near to zero) and the best optimal gain for better signal to noise ratio. Off-line analysis was performed by a single expert cardiologist. Ten randomly selected patients were reviewed by the second echocardiologist. Peak early systolic velocity (Sm), and peak early and late diastolic velocites (Em and Am) were measured and expressed in cm/second. All the measurements were calculated from three consecutive cycles, and the average of three measurements was recorded. The LV MPI defined as the sum of isovolumic contraction time (the time interval between the end of Am and the beginning of Sm) and isovolumic relaxation time (the time interval between the end of Sm and the beginning of Em) divided by ejection time (the time duration of Sm) and all data were obtained over a single cardiac cycle ( 6 ). Myocardial velocities were recorded using a standard PW-Doppler technique. (Figure1a,b). Longitudinal strain (%) is an excellent parameter for quantification of regional myocardial deformation and contractility, and strain rate (1/S) defines the rate of this deformation (17, 19). For evaluation of TD based longitudinal myocardial contraction, base of septal and lateral walls (from apical four-chamber view) and posterior wall (from three -chamber view) were analyzed (14). Peak systolic SR and late-systolic S values were averaged over three consecutive cycles for each segments (14). All data analysis performed offline on Vivid 7 GE system. A frame rate of 200–300 frames per second, and an optimal depth and angle of imaging to acquire the best resolution were used. Inter- and intra-observer variability for TDI parameters in our echocardiography lab has been previously published (19). The values are presented as mean ± SD (standard deviation) for the quantitative variables and are summarized by frequency (percentage) for the categorical variables. The comparisons between case and control groups were made using the chi-square test for the categorical data and independent two-sample t-test for continuous variables. Correlations between interval variables were measured using Pearson's correlation coefficient. For the statistical analysis,PASW Statistics 18 for Windows was used. P-value ≤ 0.05 was considered being statistically significant.3.1. Study Participants
3.2. Conventional Echocardiography
3.3. Tissue Doppler Imaging
3.4. Strain and Strain Rate Imaging
3.5. Reproducibility of Data
3.6. Statistical Analysis
4. Results
The demographic and conventional echocardiographic data of all patients and controls are summarized in Table1.
|
Table 1.
Demographic Data and Standard Echocardiographic Measurements of the Patients with Aortic Regurgitation (AR) and Healthy Control Groups
|
There was no significant difference between groups regarding age (P value = 0.555) and sex (P value = 0.264) LVEF was significantly lower and LV diameters (end systolic and end diastolic) and LV volumes (end systolic and end diastolic) were significantly higher in patients with AR compared to control group (all P values < 0.001). IVS and PW were significantly thicker (both P values < 0.001) and aorta was significantly more dilated (P value = 0.01) in patients than control participants. The mean of LA diameter was not significantly different between groups (P = 0.166). Data of PW-TDI and S/SR imaging of patients' assessment are summarized in Table2.
|
Table 2.
Comparison of pulsed wave Tissue Doppler Imaging and Strain and Strain rate imaging derived strain and strain rate parameters between patients with Aortic regurgitation (AR) and healthy control group
|
Considering PW-TDI parameters, Sm and Em of septal wall and Sm of lateral wall; Sm was significantly lower in AR patients compared to the healthy participants (all P values < 0.001) while Em and Am of septal wall did not show any significant difference between groups. IVR time was significantly longer and subsequently LV MPI significantly increased in the AR group compared to those of the healthy control group (both P-values< 0.001). With regard to the systolic longitudinal deformity indices, S and SR values of septal and lateral wall were significantly lower in AR group compared with controls (all P values < 0.05) while no significant difference was observed according to posterior wall.
5. Discussion
The present study confirmed that PW-TDI and strain and strain rate imaging methods are useful methods for early detection of the subclinical LV systolic dysfunction and could be applied as complementary echocardiography techniques for the evaluation of LV function in asymptomatic patients with chronic significant AR who had normal or preserved EF level and LV dimensions. Based on our findings, longitudinal myocardial contraction indices are significantly lower than corresponding indices in healthy participants indicating longitudinal contraction impairment in AR patients even with preserved global LVEF. LV velocity indices such as Sm and Em of septal wall, Sm of lateral wall and longitudinal peak systolic S and SR values of septal and lateral wall were significantly reduced in patients with moderate or more than moderate AR in comparison to healthy controls. Likewise, LV MPI, as a good index of evaluating combined LV systolic and diastolic functions, was significantly increased in these patients, indicating impairment in LV global function. Consistent to our findings, Sokmen et al. reported significantly reduced TDI-derived Sm of septum and anterior wall (6) and Tayyareci et al. reported significantly decreased peak systolic S and SR in each of six LV basal segment in patients with AR and preserved LV EF compared to the age and sex matched controls (8). Likewise, the mean of LV MPI values of patients were significantly increased compared to healthy participants in both of these studies. Despite the preserved LVEF level and normal LV dimensions, asymptomatic patients with chronic significant AR had lower EF level and higher LV dimensions than that of healthy controls in our study. Chronic significant AR, with a resulting volume overload leads to increasing the LV dimensions. To cope with this situation, LV hypertrophy occurs to preserve the wall stress and therefore LV contractility within the normal limits. In this stage, many patients remain asymptomatic despite developing subtle LV myocardial dysfunction (7, 8, 10, 12). Sub-endocardial ischemia as an initial stage of heart failure would firstly impair the longitodinal myocardial contraction. Without any interventions, ischemia would also influence the other myofibrils and reduce the EF level since the cardiac output is mainly dependent on shortening of the circumferential fibers (6, 9, 13-15). Whereas patients with moderate AR showed changes in S and SR that reflects the speed of deformation closely linked to the intrinsic force production. These results would propose that while total deformation is preserved, the speed of deformation is already starting to decrease even in moderate degree of regurgitation. So deformation is not directly related to the severe degree of AR (13-16, 18). Altogether, it seems that those previously established indices based on the conventional echocardiography imaging such as EF and end systolic and diastolic LV dimensions might not be sufficient to demonstrate subclinical ventricular dysfunction in these patients (15, 16). Deformation indices could provide more accurate estimation on LV function and could reflect an earlier sign of myocardial damage before that the LVEF falls and the clinical signs and symptoms of overt cardiac failure appears and thus, these complementary method to conventional echocardiography are recommended to be applied for an early determination of LV dysfunction in asymptomatic patients with significant AR and identifying patients who need surgery before developing irreversible severe heart failure (16, 17, 19). Small number of our study participants, especially in control group, and unavailability of 2D- strain/strain rate speckle imaging software were major limitations of this study. In addition, follow up of the patients group is needed to detect the small changes in the variables to the time of AVR based on the available updated guidelines to define a cutoff point for myocardial SR/S before AVR timing. Myocardial deformation study may be used as adjunctive, consistent, noninvasive parameters for evaluating subclinical ventricular dysfunction in patients with chronic significant AR. This may help to recognize patients for closer follow-up and to establish the need for surgery before developing irreversible, severe heart failure. Of course further outcome studies based on indices of global and regional deformation, are required to confirm whether reducing in deformation indices in patients with isolated AR is better index for better management strategy.
Acknowledgments
This study was Dr. Hossein Dabbaghian’s thesis to become a cardiology specialist. We would like to thank all the staff nurses in the echocardiography clinic of Tehran Heart Center, Tehran University of medical Sciences, Tehran, Iran for their kind help and cooperation during this research work.
Footnotes
References
- 1. Scognamiglio R, Negut C, Palisi M, Fasoli G, Dalla-Volta S. Long-term survival and functional results after aortic valve replacement in asymptomatic patients with chronic severe aortic regurgitation and left ventricular dysfunction. J Am Coll Cardiol. 2005;45(7):1025-30. [DOI] [PubMed]
- 2. Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84(4):1625-35. [PubMed]
- 3. Borer JS, Hochreiter C, Herrold EM, Supino P, Aschermann M, Wencker D, et al. Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation. 1998;97(6):525-34. [PubMed]
- 4. Carabello BA, Usher BW, Hendrix GH, Assey ME, Crawford FA, Leman RB. Predictors of outcome for aortic valve replacement in patients with aortic regurgitation and left ventricular dysfunction: a change in the measuring stick. J Am Coll Cardiol. 1987;10(5):991-7. [PubMed]
- 5. Chaliki HP, Mohty D, Avierinos JF, Scott CG, Schaff HV, Tajik AJ, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002;106(21):2687-93. [PubMed]
- 6. Sokmen G, Sokmen A, Duzenli A, Soylu A, Ozdemir K. Assessment of myocardial velocities and global function of the left ventricle in asymptomatic patients with moderate-to-severe chronic aortic regurgitation: a tissue Doppler echocardiographic study. Echocardiography. 2007;24(6):609-14. [DOI] [PubMed]
- 7. Lung B, Gohlke-Barwolf C, Tornos P, Tribouilloy C, Hall R, Butchart E, et al. Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J. 2002;23(16):1253-66. [PubMed]
- 8. Tayyareci Y, Yildirimturk O, Aytekin V, Demiroglu IC, Aytekin S. Subclinical left ventricular dysfunction in asymptomatic severe aortic regurgitation patients with normal ejection fraction: a combined tissue Doppler and velocity vector imaging study. Echocardiography. 2010;27(3):260-8. [DOI] [PubMed]
- 9. Di Salvo G, Pacileo G, Verrengia M, Rea A, Limongelli G, Caso P, et al. Early myocardial abnormalities in asymptomatic patients with severe isolated congenital aortic regurgitation: an ultrasound tissue characterization and strain rate study. J Am Soc Echocardiogr. 2005;18(2):122-7. [DOI] [PubMed]
- 10. Paraskevaidis IA, Kyrzopoulos S, Farmakis D, Parissis J, Tsiapras D, Iliodromitis EK, et al. Ventricular long-axis contraction as an earlier predictor of outcome in asymptomatic aortic regurgitation. Am J Cardiol. 2007;100(11):1677-82. [DOI] [PubMed]
- 11. Paraskevaidis IA, Tsiapras D, Kyrzopoulos S, Cokkinos P, Iliodromitis EK, Parissis J, et al. The role of left ventricular long-axis contraction in patients with asymptomatic aortic regurgitation. J Am Soc Echocardiogr. 2006;19(3):249-54. [DOI] [PubMed]
- 12. Vinereanu D, Ionescu AA, Fraser AG. Assessment of left ventricular long axis contraction can detect early myocardial dysfunction in asymptomatic patients with severe aortic regurgitation. Heart. 2001;85(1):30-6. [PubMed]
- 13. Tornos MP, Olona M, Permanyer-Miralda G, Herrejon MP, Camprecios M, Evangelista A, et al. Clinical outcome of severe asymptomatic chronic aortic regurgitation: a long-term prospective follow-up study. Am Heart J. 1995;130(2):333-9. [PubMed]
- 14. Marciniak A, Sutherland GR, Marciniak M, Claus P, Bijnens B, Jahangiri M. Myocardial deformation abnormalities in patients with aortic regurgitation: a strain rate imaging study. Eur J Echocardiogr. 2009;10(1):112-9. [DOI] [PubMed]
- 15. Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1-142. [DOI] [PubMed]
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-63. [DOI] [PubMed]
- 17. Smiseth OA, Edvardson T. Tissue doppler and speckle tracking echocardiography. In: Otto CM, editor(s). The practice of clinical echocardiography. Philadelphia: Saunders; 2009. p. 400-29.
- 18. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777-802. [DOI] [PubMed]
- 19. Sadeghian H, Majidi S, Lotfi-Tokaldany M, Nikdoust F, Sheikhfathollahi M, Abbasi SH. Evaluation of longitudinal tissue velocity and deformation imaging in akinetic nonviable inferobasal segments of left ventricular myocardium by dobutamine stress echocardiography. Echocardiography. 2009;26(7):801-6. [DOI] [PubMed]
 Home
Home Archive
Archive Search
Search Sign In
Sign In Site Menu
Site Menu Email this article to a friend
Email this article to a friend