This Article
Citations

Except where otherwise noted, this work is licensed under Creative Commons Attribution-NonCommercial 4.0 International License.
An Echo-Dense Cap in the Pericardial Space After Acute Myocardial Infarction: A Case Report
Abstract
Acute myocardial infarction can culminate in sudden cardiac death due to cardiogenic shock and ventricular fibrillation, and also rarely due to cardiac rupture. We present a case of post-infarction myocardial rupture after thrombolytic therapy diagnosed with transthoracic echocardiography and treated with direct closure and coronary artery bypass grafting.
Keywords: Echocardiographic Cap; Myocardial Rupture; Acute Myocardial Infraction
1. Introduction
Acute myocardial infarction (AMI) might conclude in sudden death, especially in the wake of myocardial rupture. Cardiac rupture may involve the free wall of the ventricle, interventricular septum, or papillary muscle. Ventricular free wall rupture is a significant, albeit underrecognized, cause of death after AMI. The real incidence is unknown (1). Although surgical management is crucial, the most proper surgical management remains controversial due to inadequate experience. Nonetheless, survival without surgical management appears to be infrequent (2). We describe a patient with post-infarction cardiac rupture treated with direct closure and coronary artery bypass grafting after thrombolytic therapy.
2. Case Presentation
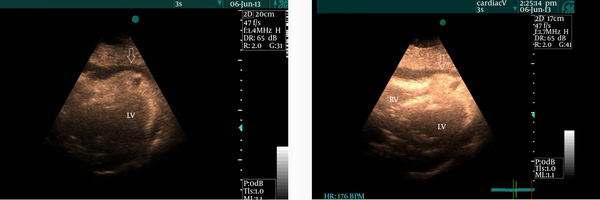
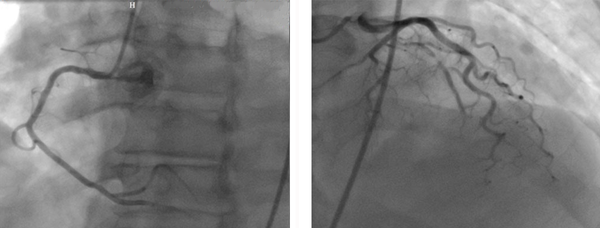
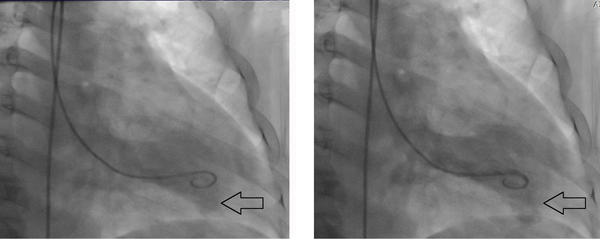
A 52-year-old man with cardiac risk factors of hypertension, diabetes mellitus, and smoking was admitted to our hospital complaining of chest pain and hyperhidrosis. On admission, the patient had a heart rate of 105 bpm and blood pressure of 95/60 mmHg. He had received thrombolytic therapy with streptokinase 24 hours previously owing to acute anterior myocardial infarction. However, he did not meet reperfusion criteria and, in addition, had a chest pain of a few hours’ duration. Echocardiography showed moderate pericardial effusion together with mild collapse of the right chambers and an echo-dense cap in the pericardial space at the cardiac apex, which was strongly suggestive of myocardial rupture (Figure 1). Cardiac catheterization revealed only a significant lesion at the mid portion of the left anterior descending artery (LAD) with normal circumflex and right coronary arteries (Figure 2). Cardiac catheterization also confirmed the myocardial rupture following a slow manual contrast injection in the left ventricle (LV), which resulted in the local opacification of the pericardial space in the apical portion (Figure 3). The patient was transferred to the operating room forthwith. The pericardium was opened, and a large volume of blood with a thrombus was removed. The hemodynamic indices improved quickly. There was active bleeding from a rupture site, approximately 4 mm in size. After the establishment of cardiopulmonary bypass, direct closure of the rupture was carried out. The left internal mammary artery bypass graft was anastomosed to the LAD. The postoperative recovery period was good and uneventful, and the patient was discharged from the hospital in good condition. Moreover, he remained asymptomatic during a 3-month postoperative follow-up.
|
Figure 1.
Subcostal view demonstrates an echo-dense cap in the pericardial space at the cardiac apex together with pericardial effusion (white arrow)
|
|
Figure 2.
Significant lesion can be seen at the mid portion of the left anterior descending artery with normal circumflex and right coronary arteries
|
|
Figure 3.
Slow manual contrast injection in the left ventricular cavity results in the local opacification of the pericardial space in the apical part (Black arrow)
|
3. Discussion
The rupture of the LV free in AMI is a rare but catastrophic complication that frequently proves fatal, with only a few patients having the chance to undergo surgery. However, survival without surgical management and repair has yet to be assessed.
Cardiac rupture in patients suffering AMI is the second most common cause of hospital death, after significant pump failure. The incidence of LV free wall rupture following AMI has been precisely defined. It complicates nearly 4% of MIs and accounts for about 12% to 21% of deaths after AMI (1, 3).
Hypertension, older age, female sex, and a first lateral or anterior wall AMI are well-known traditional risk factors for LV free wall rupture (4). The rupture occurs characteristically between 1 and 7 days after AMI. It may present abruptly with profound cardiogenic shock and cardiac tamponade (2). There might be a predilection to first MI and even single-vessel disease due to a lack of collateral vessels (5).
Sudden bradycardia and hypotension, frequently with cyanosis and also loss of consciousness, is a common indicator of impending rupture. It is triggered by the entry of blood into the pericardial cavity and is frequently transient because the following small hemopericardium acts as a tampon and prevents the further egress of blood. The onset of rupture might be heralded by chest pain, which may be very severe and resistant even to opiates, or by the classic clinical features of cardiac tamponade, shock with hypotension, quiet heart sounds, pulsus paradoxus, elevated venous pressure, bradycardia, or even electromechanical dissociation (6, 7).
Echocardiography remains the best choice for the conclusive diagnosis of LV free wall rupture. The leading echocardiographic findings in AMI patients with LV free wall rupture are pericardial effusion and intrapericardial echoes; rarely, right-heart collapse or even the actual tear itself may be seen. Importantly, echocardiography has a diagnostic sensitivity rate of 100% and a specificity rate of 93% (4).
The association between LV free wall rupture and thrombolytic treatment has been posited on the grounds that plasmin, which is activated by thrombolytic agents, has the known effect of breaking down collagen. Although the influence of thrombolysis on the incidence of myocardial rupture remains controversial, latest research shows that recently rupture occurs earlier when compared with the pre-thrombolytic era (1, 3).
Intra-aortic balloon pump support is a generally accepted management for LV septal rupture complicating MI; its role in patients with LV free wall rupture is, however, less clear. It is used uncommonly in patients with LV free wall rupture (7, 8).
LV free wall rupture can be treated with direct mattress sutures buttressed with Teflon felt with or without cardiopulmonary bypass pump. In both methods, the suture line should be along the nonischemic myocardium and transmural stitches are needed (4, 7-9).
The ease with which bleeding can be controlled in a beating heart with epicardial patching raises the question why the principle of avoiding cardiopulmonary bypass pump cannot be extended to lateral and posterior myocardial rupture via a direct approach through left thoracotomy, particularly in patients with reduced LV function (7, 10).
Therefore, the principles of the repair of LV free wall rupture are to stop the bleeding, anchor the repair on healthy tissue, minimize the distortion of the heart geometry, and revascularize if required (10). The case presented herein proves that LV free wall rupture is not always fatal and that early diagnosis and immediate surgical management may be successful.
References
- 1. Hutchins KD, Skurnick J, Lavenhar M, Natarajan GA. Cardiac rupture in acute myocardial infarction: a reassessment. Am J Forensic Med Pathol. 2002;23(1):78-82. [PubMed]
- 2. Muto A, Nishibe T, Kondo Y, Sato M, Yamashita M, Ando M. Sutureless repair with TachoComb sheets for oozing type postinfarction cardiac rupture. Ann Thorac Surg. 2005;79(6):2143-5. [DOI] [PubMed]
- 3. Sutherland FW, Guell FJ, Pathi VL, Naik SK. Postinfarction ventricular free wall rupture: strategies for diagnosis and treatment. Ann Thorac Surg. 1996;61(4):1281-5. [PubMed]
- 4. Amir O, Smith R, Nishikawa A, Gregoric ID, Smart FW. Left ventricular free wall rupture in acute myocardial infarction: a case report and literature review. Tex Heart Inst J. 2005;32(3):424-6. [PubMed]
- 5. McMullan MH, Maples MD, Kilgore TJ, Hindman SH. Surgical experience with left ventricular free wall rupture. Ann Thorac Surg. 2001;71(6):1894-8. [PubMed]
- 6. Herlitz J, Samuelsson SO, Richter A, Hjalmarson A. Prediction of rupture in acute myocardial infarction. Clin Cardiol. 1988;11(2):63-9. [PubMed]
- 7. Pretre R, Benedikt P, Turina MI. Experience with postinfarction left ventricular free wall rupture. Ann Thorac Surg. 2000;69(5):1342-5. [PubMed]
- 8. Raposo L, Andrade MJ, Ferreira J, Aguiar C, Couto R, Abecasis M, et al. Subacute left ventricle free wall rupture after acute myocardial infarction: awareness of the clinical signs and early use of echocardiography may be life-saving. Cardiovasc Ultrasound. 2006;4:46. [DOI] [PubMed]
- 9. Yip HK, Wu CJ, Chang HW, Wang CP, Cheng CI, Chua S, et al. Cardiac rupture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era. Chest. 2003;124(2):565-71. [PubMed]
- 10. Haddadin S, Milano AD, Faggian G, Morjan M, Patelli F, Golia G, et al. Surgical treatment of postinfarction left ventricular free wall rupture. J Card Surg. 2009;24(6):624-31. [DOI] [PubMed]
 Home
Home Archive
Archive Search
Search Sign In
Sign In Site Menu
Site Menu Email this article to a friend
Email this article to a friend