This Article
Citations

Except where otherwise noted, this work is licensed under Creative Commons Attribution-NonCommercial 4.0 International License.
Total Effective Radiation Dose Attributable to Medical Imaging in Patients With Acute Chest Pain: A Single-Center Comparison Study Between Dual-Source Coronary CT Angiography and Usual Care
Abstract
Introduction: Coronary CT angiography (CCTA) can safely disposition low to intermediate risk chest pain (CP); however, there is conflicting data with respect to cumulative radiation exposure when compared with usual care over short follow-up intervals.
Objectives: We report the effective radiation dose from index and downstream testing in low to intermediate risk symptomatic patients evaluated for chest pain in the ED with either CCTA or usual care to define various sources of patient radiation dose and quantify effective dose over a year and a half of follow-up.
Patients and Methods: We evaluated radiation exposure from initial and downstream testing in a prospectively collected, matched cohort evaluated for CP in the emergency department (ED) with either CCTA compared with usual care over a median follow-up of 19.6 months. Effective radiation dose was calculated using published conversion factors.
Results: Prospective, ECG-triggered acquisition using a 128-slice dual-source multidetector computed tomography (DSCT) scanner was performed in 92.9% of scans with a median effective dose from CCTA of 6.8 mSv (IQR 5.2, 9.1 mSv). CCTA cohort patients were more likely to undergo cardiac testing with exposure to radiation (P < 0.001); however, the median effective dose in patients exposed to radiation from cardiac testing was significantly lower in the CCTA cohort (7.1 mSv vs. 11.8 mSv, P < 0.001). Fewer patients in the CCTA cohort had additional non-cardiac thoracic imaging radiation exposure (40.8%) compared with usual care (92.8%). Total radiation exposure from any source was similar between the CCTA and usual care groups (100% vs 98.4%, P = 0.087), as was median total effective radiation dose (P = 0.105). Upfront CCTA was not associated with higher rates of incidental non-cardiac findings.
Conclusions: Initial evaluation of acute chest pain in the ED with CCTA was not associated with an increase in total radiation exposure over a follow-up period of 19 months. CCTA offers a more comprehensive evaluation of multiple thoracic organ systems leading to reduced radiation exposure from non-cardiac thoracic testing and no increase in incidental imaging findings. This may represent an added benefit in this population of patients presenting acutely.
Keywords: Coronary Computed Tomography Angiography; Acute Chest Pain; Effective Radiation Dose; Emergency Department
1. Introduction
Coronary computed tomography angiography (CCTA) is a well validated modality to rapidly define the presence and severity of coronary artery disease (CAD) in symptomatic patients (1). Specifically, there is an ever-growing body of data supporting implementation of CCTA to evaluate symptomatic patients presenting to the emergency department (ED) due to its very high negative predictive value (NPV) (2-5). Patients with coronary stenosis < 50% can be safely discharged home with near zero major adverse cardiovascular event (MACE) rates (2-5). Additionally, upfront CCTA in this population demonstrated a decrease in hospital admissions and a decrease in short-term healthcare costs (6). Despite the strength of this data, concern still exists that exposing patients with inherently lower risk chest pain symptoms to ionizing radiation may be unnecessary. Furthermore, many centers lack ready access to CCTA and thus rely more heavily on functional stress testing that does not involve radiation. With increasing emphasis on patient-centered imaging where patients are empowered to understand the risks and benefits of diagnostic medical imaging involving ionizing radiation, longer follow-up periods where total radiation exposure is reported is imperative (7). Most major professional societies support a linear-no-threshold model with respect to the risk of developing malignancy secondary to radiation exposure (8). Additionally, the most recent Biological Effects of Ionizing Radiation (BEIR VII) report describes a lifetime attributable risk (LAR) based on Japanese and survivors of the atomic bomb who were exposed at age 30 and survived to age 60 (9). Einstein et al. estimated lifetime attributable risk from 64-slice CCTA based on the BEIR VII model and determined the lifetime cancer risk for a 20-year old woman is 1:143 with lower attributable risk with increasing age (10) utilizing CCTA protocols with effective radiation doses between 9 - 21 mSv. This effective dose is at least 3 times greater than the average background radiation exposure from natural sources in the United States. Young women were at highest risk for breast cancer while men were more likely to develop lung cancer as a result of cardiac imaging (10). With ever improving CT scanner technologies and expertise, the effective radiation dose from CCTA continues to fall with data of sub-millisievert scan acquisitions (11, 12).
2. Objectives
We report the effective radiation dose from index and downstream testing in low to intermediate risk symptomatic patients evaluated for chest pain in the ED with either CCTA or usual care to define various sources of patient radiation dose and quantify effective dose over a year and a half of follow-up.
3. Patients and Methods
This is a retrospective analysis of a prospectively identified cohort of patients who presented to the emergency department (ED) with acute chest pain. CCTAs were acquired using a 128-slice dual-source multidetector computed tomography (DSCT) scanner between January 2013 through December 2013 at a single center, tertiary referral hospital (San Antonio military medical center, Joint Base San Antonio-Fort Sam Houston, Texas). Patients included in the rapid CCTA protocol were low-intermediate risk adults based on Thrombolysis In myocardial infarction (TIMI) risk calculator with a score of ≤ 2. Patients were evaluated for acute chest pain, as defined in prior analysis as symptoms suspicious of angina based on the ED physician’s assessment (3, 13). Onset of chest pain was within 24 hours of ED presentation with a normal or non-diagnostic electrocardiogram (ECG) without dynamic changes concerning for ischemia or injury and normal cardiac biomarkers, typically troponin T. The rapid CCTA protocol was available to the ED between 0800 hours and 1500 hours on weekdays. Exclusion criteria for the rapid CCTA protocol were patients with known CAD, elevated initial serum biomarkers, dynamic ECG changes concerning for ischemia or injury, known or suspected iodinated contrast allergy or other contraindication to receiving iodinated contrast, impaired renal function defined as a serum creatinine ≥ 1.5 mg/dL, and normal cardiac risk stratification within the preceding 12 months or a normal CCTA within the preceding 24 months. An age, gender, and cardiac risk factor matched historic cohort of patients evaluated in the ED for acute chest pain during the same time period (January 2013 through December 2013), who underwent evaluation and disposition with usual care, were retrospectively abstracted. Age-matched cohort patients met the same definition of acute chest pain as the rapid CCTA group. Patients with known CAD, definite acute coronary syndrome, or definite non-cardiac etiology of chest pain were excluded from the usual care cohort. The usual care cohort underwent evaluation in the ED without the use of CCTA. These patients were admitted to the hospital and additional diagnostic testing was performed at the discretion of the treating physicians. Available testing in the usual care cohort included exercise treadmill testing, stress echocardiography, stress myocardial perfusion imaging, stress cardiac magnetic resonance imaging, or invasive coronary angiography. All scans were analyzed by a cardiologist with level III American college of cardiology (ACC)/American college of radiology (ACR) certified imaging expertise in accordance with Society of Cardiovascular Computed Tomography (SCCT) guidelines. Scans were performed in accordance with SCCT guidelines (14, 15). All CTAs were acquired utilizing a 128-slice DSCT with a high pitch, single heart beat image acquisition capabilities (Somatom Definition Flash CT®, Siemens, Erlangen, Germany). Patients were treated prior to scan acquisition with metoprolol tartrate based on an internal protocol. Patients with heart rate (HR) < 70 bpm and systolic blood pressure (SBP) > 100 mmHg were given 50 mg orally. A 100 mg oral dose was given for HR > 70 bpm and SBP > 100 mmHg. Beta blocker was not given for patients with HR < 50 bpm or SBP < 100 mmHg at the time of evaluation. Additional oral beta blocker was administered following initial dosing at the discretion of the cardiac imaging specialist. Intravenous (IV) beta blockade was not given. All patients with SBP > 90 mmHg and without a history of phosphodiesterase inhibitor usage in the preceding 72 hours received nitroglycerin spray 0.4 - 0.8 mg within 2 minutes of scan acquisition. High-pitched, prospective, ECG-triggered helical scanning was performed with HR < 60 bpm and HR variability < 5 beats or with HR < 60 bpm at the discretion of the imaging specialist. The remaining scans were performed using prospective, ECG triggering acquisition centered at 70%-phase with 10% padding (60% - 80% of the R - R interval). Tube voltage of 120keV was used for body mass index (BMI) > 30 and was lowered to 100keV for BMI ≤ 30. CareDose® modulation of tube current was enabled for all scans. A triphasic injection protocol consisting of 75 mL of iopamidol 370 (Isovue®) IV contrast followed by 40 mL of 50% contrast mixed with normal saline chased with normal saline at a flow rate of 5 mL/second was used for all scans with total IV contrast volume of 105 - 115 mL. Contrast flow rates were increased to 6 ml/second for patients with BMI > 30. Image reconstruction was performed using iterative reconstruction. Other image interpretation parameters such as total coronary segments reviewed and non-evaluable segments (due to patient motion, cardiac motion, inadequate vessel opacification, or other artifact) were reported. CT scan protocol parameters such as tube voltage (kVp), tube current (mA), padding (msec), best diastolic phase, and total contrast volume used were also abstracted. All analysis was performed by a level III imaging cardiologist and preliminary report given to the referring ED physician within 60 minutes of image acquisition. Adjudication of CCTA was performed by a separate level III imaging cardiologist as per method, where the severity of the disease was determined on a per-patient and per-vessel basis using an 18 segment model in accordance with SCCT guidelines for interpretation (16). The major epicardial coronary arteries (left main, left anterior descending, left circumflex, and right coronary arteries) were visually graded for evidence of coronary calcification and presence and severity of coronary atherosclerosis. The posterior descending artery was included in the left circumflex or right coronary artery groups depending on its origin. We categorized these patients based upon the severity of CAD, defined as obstructive CAD (> 50% stenosis), non-obstructive CAD (≤ 50% stenosis), or no CAD (vessels free of angiographic evidence of disease). Additionally, segment involved score (SIS) and sum stenosis score (SSS) was calculated as previously described (17). All medical imaging studies performed on patients in both cohorts were abstracted using a local electronic medical records (EMR) system in order to define the magnitude of radiation dose attributable to various types of imaging. Individual patient imaging studies were divided into three categories: cardiac imaging, non-cardiac thoracic imaging, and non-thoracic imaging. Cardiac imaging included all ischemic and anatomic cardiac testing available at our institution, which includes invasive coronary angiography (ICA), single-photon emission computed tomography (SPECT), positron emission tomography (PET), CCTA, stress echocardiography, and treadmill exercise stress testing. Non-cardiac imaging included chest CT scans both with and without IV contrast, CT pulmonary angiography, ventilation-perfusion (VQ) scans, plain chest radiographs (CXR), and lung PET scans. Non-thoracic imaging included all other diagnostic or therapeutic imaging scans performed on the patients during the follow-up after ED chest pain evaluation. Non-thoracic imaging was included in order to estimate the total per-patient radiation exposure over the follow-up period and to lend perspective to the proportion of per-patient radiation attributable to cardiac and thoracic imaging. Diagnostic and therapeutic procedures utilizing fluoroscopy, with the exception of ICA, were excluded as information needed to calculate effective dose was not readily available for review. Incidental imaging findings to include pulmonary nodules, pulmonary embolism, lymphadenopathy, pneumonia, pneumothorax, pleural effusion, and bone lesions were also abstracted. Electronic medical record (EMR) was queried for all index and downstream testing performed on the study patients. Any testing performed on the day of ED evaluation for the usual care cohort or the date of CCTA acquisition for the ED CCTA cohort was used as the index evaluation. Medical imaging involving ionizing radiation was broken up into cardiac imaging, non-cardiac thoracic imaging, and non-thoracic imaging. Calculation of effective radiation dose was performed using previously published standards (3, 18). The dose length product (DLP) for CCTA to include coronary artery calcium score (CAC), if it was performed, using an organ weighting factor of k = 0.014 mSv mGy-1 cm-1 was used to convert from DLP to effective dose in mSv. Effective doses from CT chest, abdomen and pelvis, and head were calculated using DLP and organ weighting factors of 0.014 mSv mGy-1 cm-1, 0.017 mSv mGy-1 cm-1, and 0.0023 mSv mGy-1 cm-1, respectively. Single-photon emission computed tomography (SPECT) and positron emission tomography (PET) effective radiation dose was calculated using the conversion 1 millicurie (mCi) is equal to 37 megabecquerel (MBq) (1 MBq = 0.0085 mSv). Radiation exposure from ICA was calculated by converting air kerma to a dose-area product (DAP) and applying the conversion factor of 0.22 mSv/Gy cm2 (18). The primary endpoint was total effective dose secondary to any medical imaging between the CCTA and usual care groups. Additional primary endpoints included determination of the effective dose secondary to sub-categories of medical imaging (cardiac, non-cardiac thoracic, and non-thoracic) and the incidence of radiation exposure in each cohort. Secondary endpoints included evaluation of effective dose received based on CAD burden and other baseline demographic factors. The incidence of non-cardiac incidental findings between the groups was also abstracted. Age- and risk factor-matched cohort was obtained using propensity scoring utilizing categorical matching on usual care ED patients evaluated for chest pain syndromes during the same time period. Statistical analysis was performed using IBM SPSS version 19.0 (IBM, Armonk, New York). Continuous variables are presented as means ± standard deviation and medians with interquartile range (IQR) or ranges, as appropriate. Categorical variables are presented as frequencies with percentages. Comparison of mean and median values was performed using one-way ANOVA with post-hoc Bonferroni correction or Mann-Whitney U test, respectively. Kruskal-Wallis test was used to compare median values when appropriate. P-values less than 0.05 were considered significant. The authors declare that this study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our local institution’s human research committee.3.1. Patient Selection
3.2. CCTA Cohort
3.3. Usual Care Cohort
3.4. Noninvasive Coronary Artery Analysis by CCTA
3.5. CCTA Stenosis Severity Assessment
3.6. Medical Imaging
3.7. Effective Radiation Dose
3.8. Study Endpoints
3.9. Statistical Analysis
4. Results
A total of 366 patients presenting to the ED with acute chest pain were analyzed with 182 patients evaluated using CCTA in the ED and 184 patients evaluated in a usual fashion. The median follow-up for the entire cohort was 19.6 months. Table 1 summarizes the baseline demographic data for the entire population, as well as the CCTA and usual care cohorts. The median age at the time of evaluation was 48 years (IQR 42, 57 years) and there were no significant differences between the groups with respect to age, gender, body mass index (BMI), CAD risk factors, nor TIMI risk scores.
|
Table 1.
Baseline Demographic Data and Effective Radiation Dosea
|
Specifics of the CCTA protocols used in the CCTA cohort are outlined in Table 2. All patients were imaged using prospective, ECG-triggered axial imaging (92.8%) or prospective, ECG-triggered high-pitched helical (8.2%) acquisitions. Both CCTA and CAC scores were obtained in 91.2% of patients. A median patient BMI in the CCTA cohort of 30.1 lb/in2 (IQR 26.0, 33.9 lb/in2) resulted in 96.7% of patients being scanned utilizing a tube voltage of 120 kVp. Median tube current was 786 mA (IQR 630, 929 mA) and median padding was 615 ms (IQR 503, 686 ms). The vast majority of CCTAs resulted in diagnostic image quality with a modest 3.6% of the segments being determined not interpretable.
|
Table 2
. CCTA Cohort Detailsa
|
Obstructive CAD (≥ 50% stenosis) was present in 6%, nonobstructive CAD (1% - 49% stenosis) was present in 35.7%, and CAD was absent (no stenosis, CAC of 0) in 58.2%. In the CCTA cohort, obstructive CAD was confirmed in 3 patients (75% true positive) by ICA. All three of these patients proceeded to revascularization (2 via percutaneous coronary intervention (PCI) and 1 via coronary artery bypass grafting (CABG). Revascularization was deferred in the fourth patient following a non-significant fractional flow reserve (FFR) of 0.86. Nine ICAs were performed in 8 patients in the usual care cohort. Of the 9 ICAs, 5 were found to have no significant CAD, 2 patients had non-obstructive CAD (stenosis < 70%), and 2 patients underwent PCI for single vessel obstructive CAD (22% true positives).
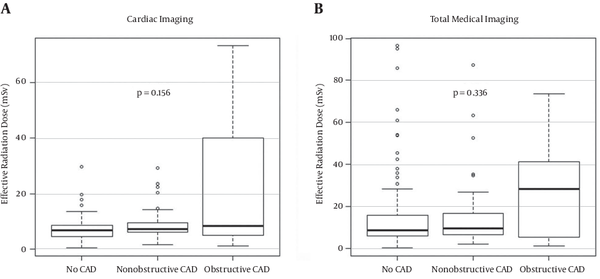
Median effective dose attributable to CCTA was 6.0 mSv (IQR 4.4, 8.0 mSv), CAC was 0.85 mSv (IQR 0.69, 1.1 mSv), and total scan was 6.8 mSv (IQR 5.2, 9.1 mSv). There was no difference in effective radiation dose from cardiac imaging (Figure 1 A) between patients with obstructive CAD (median 8.4 mSv (IQR 4.9, 40.0 mSv) compared with nonobstructive CAD and no CAD (median 7.1 mSv (IQR 6.0, 9.4 mSv) and 6.7 mSv (IQR 4.6, 8.5 mSv), P = 0.156). Additionally, median total radiation dose (Figure 1B) did not differ significantly based on CAD burden (P = 0.336).
|
Figure 1.
Box Plot Depicting Median Effective Radiation Dose Attributable to A, Cardiac Imaging and B, Total Medical Imaging in the CCTA Cohort as a Function of Coronary Artery Disease (CAD) Burden
|
Total median effective radiation dose (Table 1) over the 19.6 month follow-up was not significantly different in the CCTA cohort (9.1 mSv (IQR 6.2, 17.0) compared with the usual care cohort (13.1 mSv (IQR 4.7, 29.2), P = 0.105). Likewise, there was no difference between the groups with respect to the total percentage of patients who underwent any diagnostic testing involving radiation (CCTA 100% vs usual care 98.4%, P = 0.087). More patients in the CCTA cohort (Table 1) underwent cardiac testing (55.6% vs 47.2%, P = 0.025). Patients in the CCTA cohort were also more likely to undergo cardiac testing involving ionizing radiation (100% vs 55.2%, P < 0.001). However, of the patients in each group receiving cardiac testing involving radiation, the median effective dose was lower in patients in the CCTA arm (median: 7.1 mSv (IQR 5.3, 9.3) vs median 11.8 mSv (6.0, 13.7), P < 0.001). By comparison, patients in the usual care cohort were far more likely to undergo non-cardiac thoracic imaging (Table 1) than patients initially evaluated with CCTA (77.8% vs 22.2%, P < 0.001). A significant increase was also observed in the percentage of patients receiving ionizing radiation from a non-cardiac thoracic test in the usual care cohort (92.8% vs 40.8%, P < 0.001), though the median effective radiation dose was modest in both groups (usual care 0.18 (IQR 0.06, 11.2) vs CCTA 0.06 (IQR 0.06, 0.18), P < 0.001). Despite modest median radiation dose, 25.1% of the total effective radiation dose in the usual care cohort was attributable to non-cardiac thoracic imaging compared with 5.6% in the CCTA cohort (P < 0.001).
Table 3 summarizes effective radiation dose grouped by age. There was no significant difference between the 2 groups in the percentage of patients in their 30s, 40s, 50s, 60s, or 70s that were exposed to any amount of radiation due to medical imaging. Excluding patients in their 70s, CCTA cohort patients in all of these age groups were more likely to receive radiation from a cardiac imaging source (P < 0.001 in all age groups). Patients in their 40s and 60s in the CCTA cohort received a lower median dose from cardiac testing than usual care (P = 0.003 and P = 0.001, respectively). No significant difference in total effective radiation dose was observed between the 2 groups.
|
Table 3.
Effective Radiation Dose and Radiation Exposure by Decade of Agea
|
Incidental findings (Table 4) associated with all index and downstream testing were not significantly different between the groups with the exception of incident bony lesions. Bone lesions were more commonly observed incidentally in patients in the usual care cohort (2.7% vs 0%, P = 0.022). Additionally, 3 patients in the CCTA cohort were diagnosed with alternate, non-cardiac etiologies of chest pain due to CCTA (2 patients with pneumonia and 1 patient with a pulmonary embolus).
|
Table 4.
Incidence of Incidental Imaging Findingsa
|
5. Discussion
A strategy of CCTA using DSCT in the ED to evaluate low to intermediate risk acute chest pain patients was not associated with an increase in the incidence of radiation exposure nor total median radiation dose when compared with a propensity matched, usual care cohort over a follow-up period of 19 months. Additionally, the median effective dose secondary to cardiac imaging was lower in the CCTA cohort despite this cohort being comprised primarily of overweight patients (median BMI 30.1 lb/in2) and predominantly utilizing 120 kVp imaging protocols (96.7%). Additional thoracic imaging was more commonly obtained in the usual care cohort accounting for the vast majority of effective radiation dose received in this cohort. These findings, coupled with a previously published analysis from this same population demonstrating that use of CCTA in the ED was associated with more rapid ED disposition times (median 5.9 hours vs. 25.0 hours, P < 0.001), lower hospital admission rates (9.3% vs 98.9%, P < 0.001), and lower total payer cost ($ 182,064.55 vs $ 685,190.77, P < 0.001) with no increased risk to patients, strengthens the argument for the routine use of CCTA in the ED (5). This finding may be explained by the fact that CCTA is a more comprehensive testing modality for the evaluation of acute chest pain patients in the ED. In addition to being the most sensitive modality for the detection of CAD, the scan volumes routinely include the vast majority of the lung fields and routinely allow for visualization of the proximal pulmonary arteries and a large portion of the thoracic aorta.
Large randomized trials utilizing a CCTA strategy for the evaluation of acute CP report mixed findings with respect to radiation exposure and downstream testing over short term follow-up. The rule out myocardial infarction using computer assisted tomography (ROMICAT) II randomized 1000 patients with possible acute coronary syndrome (ACS), a non-ischemic ECG, and negative cardiac biomarkers to CCTA or standard evaluation in the ED (2). Over a relatively short follow-up period of 28 days, CCTA reduced ED length of stay and increased ED patient discharge with no difference in adverse cardiovascular outcomes. Similar to our findings, these authors reported an increase in radiation exposure (97% vs 33% with standard evaluation), but lower mean effective dose in the CCTA group (11.3 mSv for CCTA vs 14.1 mSv for SPECT, P < 0.001). In ROMICAT II, over 70 patients were imaged using 128-slice DSCT technology resulting in half of the effective radiation dose when compared to those who were imaged using older technology (6.2 ± 3.8 mSv vs 12.3 ± 5 mSv). Thus, as CT scanner technology continues to improve, the effective radiation dose gap between SPECT and cardiac CT is likely to grow.
Observational data from the same group in ROMICAT I found that over 40% of patients with no CAD and over 51% of patients with non-obstructive CAD by CCTA (performed in a blinded fashion prior to admission) underwent evaluation with either SPECT imaging or ICA with the associated procedural risk and significantly higher effective radiation dose (13, 19). Thus, in centers where CCTA is not available, a large portion of patients without significant CAD are undergoing unnecessary invasive evaluation or work-up with a less sensitive modality with a higher effective radiation dose than that offered by CCTA. Recently published data from our group suggests the absence of obstructive CAD on CCTA (defined as stenosis ≥ 50%) resulted in lower downstream cost and utilization of additional ischemic testing during subsequent chest pain evaluations (20). This is likely explained by the recent findings from SCOT-HEART where CCTA increased diagnostic certainty when compared to clinical evaluation in combination with exercise stress testing (21). Thus, upfront radiation exposure from CCTA may be offset by reduced downstream radiation exposure over a longer term follow-up period, particularly in patients with no and non-obstructive CAD.
Similar to previously published data, our CCTA cohort was more likely to receive radiation secondary to cardiac imaging with lower median effective radiation dose attributable to cardiac imaging. Some data suggest increased rates of incidental, non-cardiac findings that do not assist in diagnosis but add to downstream testing and radiation exposure. However, we observed no difference in the incidence of these findings between the cohorts (22). Finally, the vast majority of radiation exposure from cardiac imaging occurred in patients age 40 and 60 years, with patients in the youngest decade of this range having a significant reduction in median effective radiation dose. While reduction in radiation dose and exposure may not affect downstream cancer risk as dramatically in this group when compared to patients in their 3rd decade of life, current guidelines recommend minimizing dose and exposure regardless of age. Given the very good prognosis in CCTA patients with no or minimal CAD over 5-6 years of follow-up, perhaps longer term follow-up is needed before the full benefit of an index CCTA is realized (23-25).
Study Limitations: This is a single center analysis involving a relatively small sample size enrolled in a closed-referral healthcare system, thus the findings may not be generalizable to an open referral health system. The use of an organ weighting factor of 0.014 mSv mGy-1 cm-1, though used as a standard clinical conversion factor, is reported to underestimate effective dose of CCTA by up to 50%, thus actual CCTA per-patient doses may be higher than calculated (26-28). Effective radiation doses from procedures involving fluoroscopy (either interventional radiology or at the time of a surgical operation) were not included in this analysis as the air kerma levels were not available. The use of DSCT imaging is not readily available in all ED hospitals, thus the reported effective radiation doses may not be generalizable. Given the fact that the patients in the CCTA and usual care cohorts were evaluated during the same time period, it is likely that the majority of patients in the usual care cohort presented on weekends and after normal business hours when CCTA was not available.
5.1. Conclusion
In this single center, initial evaluation of acute chest pain in the ED with CCTA was not associated with an increase in total radiation exposure over a follow-up period of 19 months. CCTA offers a more comprehensive evaluation of multiple thoracic organ systems leading to reduced radiation exposure from non-cardiac thoracic testing and no increase in incidental imaging findings. This may represent an added benefit in this population of patients presenting acutely.
Footnotes
References
- 1. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122(21):e525-55. [DOI] [PubMed]
- 2. Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308. [DOI] [PubMed]
- 3. Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-22. [DOI] [PubMed]
- 4. Cury RC, Budoff M, Taylor AJ. Coronary CT angiography versus standard of care for assessment of chest pain in the emergency department. J Cardiovasc Comput Tomogr. 2013;7(2):79-82. [DOI] [PubMed]
- 5. Jones RL, Thomas DM, Barnwell ML, Fentanes E, Young AN, Barnwell R, et al. Safe and rapid disposition of low-to-intermediate risk patients presenting to the emergency department with chest pain: a 1-year high-volume single-center experience. J Cardiovasc Comput Tomogr. 2014;8(5):375-83. [DOI] [PubMed]
- 6. Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, et al. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;61(8):880-92. [DOI] [PubMed]
- 7. Einstein AJ, Berman DS, Min JK, Hendel RC, Gerber TC, Carr JJ, et al. Patient-centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol. 2014;63(15):1480-9. [DOI] [PubMed]
- 8. Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5(4):198-224. [DOI] [PubMed]
- 9. Nuclear and Radiation Studies Board DoEaLS . Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation.. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington: The National Academies Press; 2006.
- 10. Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-23. [DOI] [PubMed]
- 11. Schuhbaeck A, Achenbach S, Layritz C, Eisentopf J, Hecker F, Pflederer T, et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur Radiol. 2013;23(3):597-606. [DOI] [PubMed]
- 12. Layritz C, Muschiol G, Flohr T, Bietau C, Marwan M, Schuhbaeck A, et al. Automated attenuation-based selection of tube voltage and tube current for coronary CT angiography: Reduction of radiation exposure versus a BMI-based strategy with an expert investigator. J Cardiovasc Comput Tomograph. 2013;7(5):303-10. [DOI]
- 13. Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53(18):1642-50. [DOI] [PubMed]
- 14. Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, et al. SCCT guidelines for performance of coronary computed tomographic angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomograph. 2009;3(3):190-204. [DOI]
- 15. Raff GL, Chinnaiyan KM, Cury RC, Garcia MT, Hecht HS, Hollander JE, et al. SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: A Report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomograph. 2014;8(4):254-71. [DOI]
- 16. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342-58. [DOI] [PubMed]
- 17. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161-70. [DOI] [PubMed]
- 18. Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116(11):1290-305. [DOI] [PubMed]
- 19. Hulten E, Goehler A, Bittencourt MS, Bamberg F, Schlett CL, Truong QA, et al. Cost and resource utilization associated with use of computed tomography to evaluate chest pain in the emergency department: the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) study. Circ Cardiovasc Qual Outcomes. 2013;6(5):514-24. [DOI] [PubMed]
- 20. Thomas DM, Shaw DJ, Barnwell ML, Jones RL, Ahmadian HR, Prentice RL, et al. The lack of obstructive coronary artery disease on coronary CT angiography safely reduces downstream cost and resource utilization during subsequent chest pain presentations. J Cardiovasc Comput Tomograph. 2015;9(4):329-36. [DOI]
- 21. investigators SH. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383-91. [DOI] [PubMed]
- 22. Dewey M, Schnapauff D, Teige F, Hamm B. Non-cardiac findings on coronary computed tomography and magnetic resonance imaging. Eur Radiol. 2007;17(8):2038-43. [DOI] [PubMed]
- 23. Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Bertella E, Antonioli L, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 2012;5(7):690-701. [DOI] [PubMed]
- 24. Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, et al. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation. 2012;126(3):304-13. [DOI] [PubMed]
- 25. Hadamitzky M, Taubert S, Deseive S, Byrne RA, Martinoff S, Schomig A, et al. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34(42):3277-85. [DOI] [PubMed]
- 26. Einstein AJ, Elliston CD, Arai AE, Chen MY, Mather R, Pearson GD, et al. Radiation dose from single-heartbeat coronary CT angiography performed with a 320-detector row volume scanner. Radiology. 2010;254(3):698-706. [DOI] [PubMed]
- 27. Geleijns J, Joemai RM, Dewey M, de Roos A, Zankl M, Cantera AC, et al. Radiation exposure to patients in a multicenter coronary angiography trial (CORE 64). AJR Am J Roentgenol. 2011;196(5):1126-32. [DOI] [PubMed]
- 28. Goetti R, Leschka S, Boschung M, Mayer S, Wyss C, Stolzmann P, et al. Radiation doses from phantom measurements at high-pitch dual-source computed tomography coronary angiography. Eur J Radiol. 2012;81(4):773-9. [DOI] [PubMed]
 Home
Home Archive
Archive Search
Search Sign In
Sign In Site Menu
Site Menu Email this article to a friend
Email this article to a friend